at the University of Wisconsin, Madison
References
SELECTED BIBLIOGRAPHY OF ISRU PUBS By Lawrence A. Taylor
- Taylor, L.A., 1987, Rocks and minerals of the Moon: Materials for a lunar base. Symposium ’86, S86-37, 20p.
- Taylor, L.A., 1988, Generation of native Fe in lunar soil. Proc. Space 88, Amer. Soc. Civil Engr., 67-77.
- Taylor, L.A., 1990, Hydrogen, helium, and other solar-wind components in lunar soil: Abundances and predictions. Proc. Space 90, Amer. Soc. Civil Engr., 68-77.
- Oder, R.R., and L.A. Taylor, 1990, Magnetic beneficiation of highland and hi-Ti mare soils: Magnet requirements. Proc. Space 90, Amer. Soc. Civil Engr., 133-142.
- Taylor, L.A., and R.R. Oder, 1990, Magnetic beneficiation of highland and hi-Ti mare soils: Rock, mineral, and glassy components. Proc. Space 90, Amer. Soc. Civil Engr., 143-152.
- Papike, J.J., L.A. Taylor, and S. Simon, 1991, Lunar minerals. In Lunar Sourcebook, Heiken, Vaniman, and French (eds.), Cambridge Univ. Press, Chapter 5, 121-181.
- Taylor, L.A., B. Cooper, D.S. McKay, and R.O. Colson, 1991, Oxygen production on the Moon: Processes for different feedstocks. In Advanced Materials – Applications of Mining and Metallurgical Processing Principles, Publ. of Soc. Min., Metal., and Explor. (SME), 29-45.
- Taylor, L.A., and W.D. Carrier, III, 1992, The feasibility of processes for the production of oxygen on the Moon. In Engineering, Construction, Operations in Space III, Vol. I, Eds. Sadeh, Sture and Miller, ASCE, New York, 752-762.
- Taylor, L.A., and D.S. McKay, 1992, Beneficiation of lunar rocks and regolith: Concepts and difficulties. In Engineering, Construction, Operations in Space III, Vol. I, Eds. Sadeh, Sture and Miller, ASCE, New York, 1058-1069.
- Taylor, L.A., 1992, Planetary science and resource utilization at a lunar outpost: Chemical analytical facility requirements. In A Lunar-Based Chemical Analysis Laboratory, Proceedings of the Ninth College Park Colloquim on Chemical Evolution, Eds. Ronnamperu ma and Gehrke, Hampton, 111-131.
- Taylor, L.A., 1992, Resources for a lunar base: Rocks, minerals, and soil of the moon. In The Second Conference on Lunar Bases and Space Activities of the 21st Century, NASA Publ. 3166, Vol. 2, 361-377.
- Taylor, L.A., and F. Lu, 1992, The formation of ore mineral deposits on the Moon: A feasibility study. In The Second Conference on Lunar Bases and Space Activities of the 21st Century, NASA Publ. 3166, Vol. 2, 379-383.
- Taylor, L.A., 1992, Production of oxygen on the Moon: Which processes are best and why. Amer. Inst. Aero. Astro. Space Programs & Tech. Confer., AIAA, 92-1662, 1-9.
- Taylor, L.A. and W.D. Carrier, III, 1992, Production of oxygen on the Moon: Which processes are best and why. AIAA Journal, Vol. 30, no. 12, 2858-2863.
- Taylor, L.A., and W.D. Carrier, III, 1993, Oxygen production on the moon: An overview and evaluation. Chapter in Resources of Near-Earth Space, Univ. of Ariz. Series, 69-108.
- Chamberlain, P.G., L.A. Taylor, E.R. Podnieks, and R.J. Miller, 1993, A review of possible mining applications in space. Chapter in Resources of Near-Earth Space, Univ. of Ariz. Series, 51-68.
- Chambers, J.G., L.A. Taylor. A. Patchen, and D.S. McKay, 1994, Mineral liberation and beneficiation of lunar high-Ti mare basalt, 71055: Digital-imaging analyses. In Engineering, Construction, Operations in Space IV, Vol.II, ASCE, New York, 878-888.
- Taylor, L.A., 1994, Evidence of helium-3 on the Moon : Model assumptions and abundances, In Engineering, Construction, Operations in Space IV, Vol. II, ASCE, New York, 678-686.
- Chambers, J.G., L.A. Taylor, A. Patchen, and D.S. McKay, 1995, Quantitative mineralogical characterization of lunar high-Ti mare basalts and soils for oxygen production. J. Geophys. Res.-Planets, 100, E7, 14,391-14,401.
- Higgins, S.J., L.A. Taylor, A. Patchen, J. Chambers, and D.S. McKay, 1996, X-ray digital imaging petrography:Technique development for lunar mare soils. Meteoretics & Planet. Sci., 31, 356-361.
- Taylor, L.A. and D.S. Taylor, 1996, Location of a lunar base:A site selection strategy, In Engineering, Construction, Operations in SpaceV, Vol. III, ASCE, New York, 741-755.
- Taylor, L.A., A. Patchen, D.-H. S. Taylor, J.G. Chambers, and D.S. McKay, 1996, X-ray digital imaging and petrography of lunar mare soils:Data input for remote sensing calibrations, Icarus 124, 500-512..
- Taylor, L.A., and D.-H. Taylor, 1997, Considerations for a return to the Moon and Lunar Base site selection workshops. Jour. Aerosp. Engr. 10, 68-79.
- Taylor, L.A., 1997, Resource utilization at a lunar outpost. In A Lunar-Based Analytical Laboratory, Deepak Publ., Hampton, VA, 99-107.
- Taylor, L.A., 1997, Oxygen and water productions on the Moon, In A Lunar-Based Analytical Laboratory, Deepak Publ., Hampton, VA, 56-63.
- Taylor, L.A., and Kulcinski, G.L., 1999, Helium-3 on the Moon for Fusion Energy: The Persian Gulf of the 21st Century. Solar System Research 33, 338-345.
- McKay, D.S., and Taylor, L.A., 1999, Lunar resource utilization. In Lunar Base Handbook, (editor, P. Eckart), McGraw-Hill, 603-606.
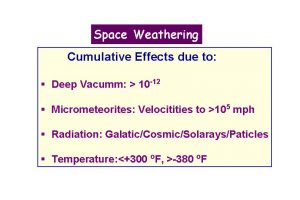
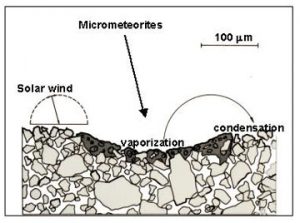
- Taylor, L.A., Pieters, C., Keller, L.P., Morris, R.V., McKay, D.S., Patchen, A., and Wentworth, S., 2000, Space weathering of lunar mare soils: New understanding of the effects of reflectance spectroscopy. Space 2000, Amer. Soc. Civil Engr., 703-711.
- Pieters, C.M., Taylor, L.A., Noble, S.K., Keller, L.P., Hapke, B., Morris, R.V., Allen, C.C., McKay, D.S., and Wentworth, S., 2000, Space weathering on airless bodies: Resolving a mystery with lunar samples. Meteor. Planet. Sci. 35, 1101-1107.
- Taylor, L.A., Pieters, C., Keller, L.P., Morris, R.V., McKay, D.S., Patchen, A., and Wentworth, S., 2001, The effects of space weathering on Apollo 17 mare soils: Petrographic and chemical characterization. . Meteor. Planet. Sci. 36, 285-299.
- Noble, S.K., Pieters, C.M., Taylor, L.A., Morris, R.V., Allen, C.C., McKay, D.S., and Keller, L.P., 2001, The optical properties of the finest fraction of lunar soil: Implications for space weathering. . Meteor. Planet. Sci. 36, 31-42.